Asphondylia xerezi Maia, 2024
|
publication ID |
https://doi.org/10.11606/1807-0205/2024.64.008 |
|
publication LSID |
lsid:zoobank.org:pub:C7DC7A88-F5D8-4356-9E9C-85994FFDE18F |
|
persistent identifier |
https://treatment.plazi.org/id/C4474D20-695D-FFE0-F9FF-F843FA43F94F |
|
treatment provided by |
Felipe |
|
scientific name |
Asphondylia xerezi Maia |
| status |
sp. nov. |
Asphondylia xerezi Maia , sp. nov.
( Figs. 19 View Figure 19 A-21C)
Diagnosis: Male hypoproct rounded apically, deeply bilobed, ovipositor with needle part about 2.45 × length 7 th sternite; pupa: antennal horn 0.20-0.25 mm long, upper facial horn single and conical, three lower frontal horns not aligned, 8 th abdominal segment with 7-10 dorsal spines in the posterior row, larva: spatula with lateral and mesal teeth subequal in length, mesal teeth round- ed apically, three setose lateral papillae on each side of spatula.
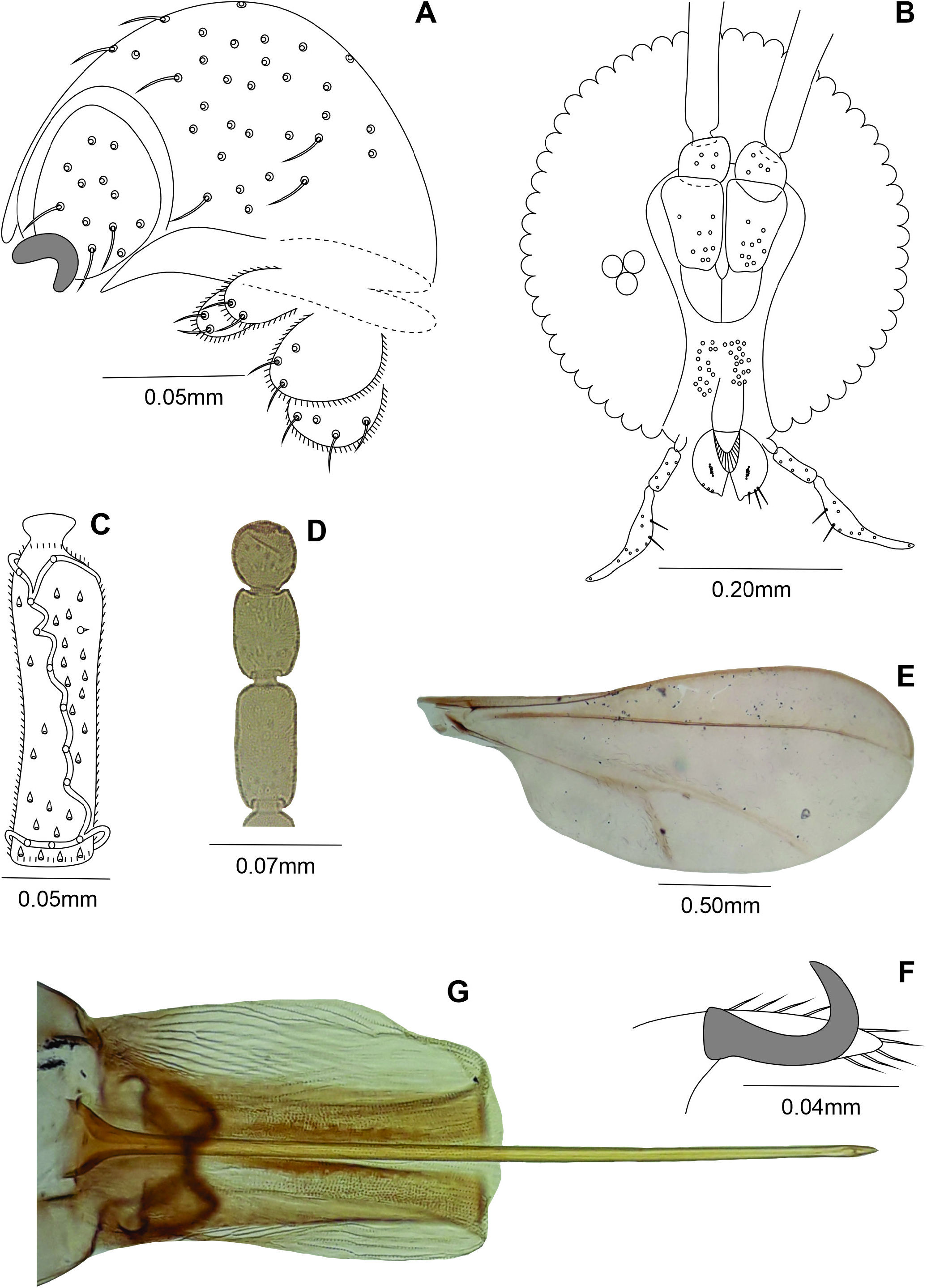
Male: Body: 3.20 mm long (N = 1). Head: 0.45 mm long, 0.45 mm wide (N = 1), eye facets circular, closely appressed; antennae: flagellomeres 1 and 2 not fused, scape obovate, setose, 0.06 mm long, 0.06 mm wide (N = 1), pedicel globose, setose, 0.05 mm long, 0.06 mm wide (N = 1), 1 st- 7 th flagellomeres cylindrical, all 0.04 mm wide (8 th to 12 th flagellomeres missing), circumfila longitudinally wavy, dense, anastomosing, equally spread along segments, 1 st flagellomeres 0.22 mm long (N = 1), 2 nd- 7 th flagellomeres 0.17-0.18 mm long (N = 1), proportion flagellomere neck-node 1:09; frons smashed; mouthparts smashed. Thorax: scutum with two dorsocentral rows of setae, setae more abundant anteriorly, two groups of lateral setae more abundant anteriorly, extending from base to distal margin, scales intermixed; scutellum with scattered setae; anepimeron setose; remaining pleural sclerites bare; legs: tarsal claws curved beyond midlength, empodium as long as claws; wing smashed. Abdomen: trichoid sensilla not visible; 1 st- 7 th tergites sclerotized, rectangular with a posterior row of setae, few scattered lateral setae and mostly covered elsewhere with scales, 8 th tergite tergite8 th band-like, bare; 2 nd- 8 th sternites sclerotized, rectangular, narrower than tergites, with a posterior row of setae, several setae and midlength, few lateral setae, and mostly covered elsewhere with scales; 8 th sternite with setae at ⅔ distal and mostly covered elsewhere with scales. Terminalia ( Fig. 19A View Figure 19 ): gonocoxite short and stout, 0.15 mm long, 0.07 mm wide (N = 1); gonostylus ovoid, 0.06 mm long, 0.05 mm wide (N = 1); hypoproct deeply bilobed, rounded apically.
Female: Body length: 3.20 mm (N = 1). Head ( Fig. 19B View Figure 19 ): 0.40 mm long, 0.35 mm wide, antennae: scape 0.09 mm long, 0.05 mm wide, pedicel 0.05 mm long, 0.05 mm wide, 1 st- 11 th flagellomeres cylindrical, all 0.04 mm wide, circumfila comprising two longitudinal bands connected subbasally and apically by two transverse bands ( Fig. 19C View Figure 19 ), flagellomeres 1 and 2 not fused, 1 st flagellomere 0.24 mm long, 2 nd- 6 th flagellomeres 0.17-0.18 mm long, 7 th flagellomere 0.15 mm long (N = 4), 8 th flagellomere 0.14 mm long (N = 1), 9 th flagellomere 0.09 mm long (N = 1), 10 th flagellomere 0.07 mm long, 11 flagellomere 0.06 mm long, 12 th flagellomere 0.04 mm long ( Fig. 19D View Figure 19 ); proportion flagellomere neck-node 1:15; mouthparts: labrum 0.07 mm long, 0.04 mm wide (N = 1), hypopharynx 0.11 mm long, 0.04 mm wide (N = 1), labellum 0.07 mm long, 0.04 mm wide at midlength, with 5 pairs of mesal setae (N = 1); palpus 0.22 mm long (N = 1): 1 st segment globose 0.02 mm long, 0.02 mm wide (N = 2), 2 nd segment cylindrical 0.05 mm long, 0.02 mm wide at midlength (N = 2), 3 rd segment claviform 0.15 mm long and 0.02-0.03 mm wide at midlength (N = 2). Thorax: wing length: 2.35 mm (N = 1) ( Fig. 19E View Figure 19 ); tarsal claws more sclerotized and robust than in male ( Fig. 19F View Figure 19 ). Abdomen: trichoid sensillae not visible, 1 st- 7 th tergites as in male, 8 th tergite with posterior margin with lobes 0.07-0.08 mm long (N = 2), 2 nd- 6 th sternites as in male, 6 th sternite 0.17-020 mm long (N = 2), 7 th sternite 0.35-45 mm long (N = 1), 2.05-2.25 × length sternite 6 (N = 2), setose (except basally), mostly covered elsewhere with scales; sternite 8 not sclerotized; ovipositor ( Fig. 19G View Figure 19 ): needle part 0.86 mm long (N = 1), 2.45x length sternite 7 (N = 1). Other characters as in male.
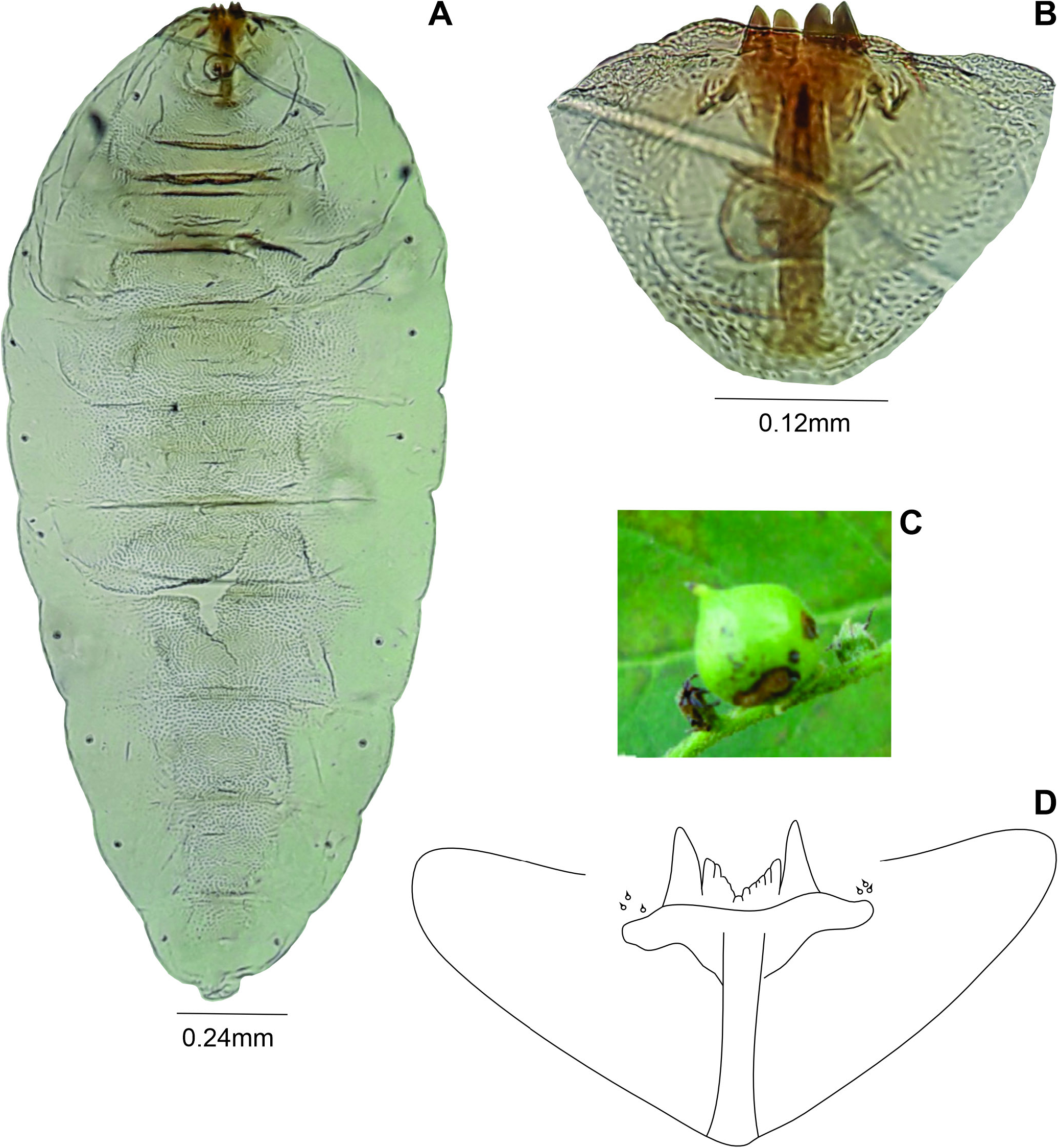
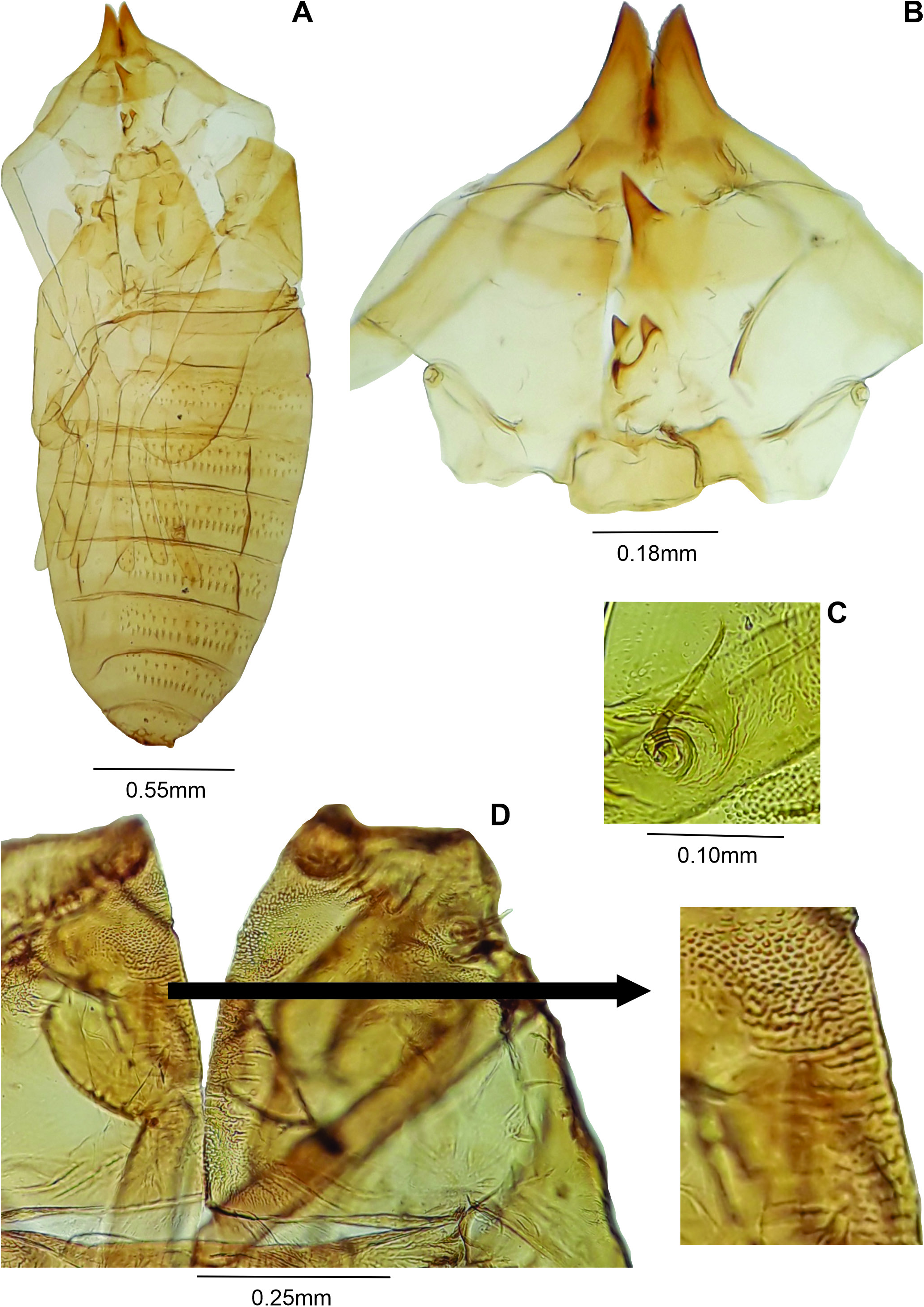
Pupa ( Fig. 20A View Figure20 ): Color: brownish. Body length: 2.90-3.20 mm (N = 3). Head ( Fig. 20B View Figure20 ): dorsal plate 0.38-0.40 mm long, 0.18-0.20 mm wide (N = 2); antennal horn 0.20-0.25 mm long (N = 3), conical, pointed, distal part longer than basal part, inner margin serrated; dorsal plate 0.20 mm long, 0.39-0.40 mm wide (N = 3), apical seta 0.05 mm long (N = 3); one upper facial horn conical, 0.07-0.11 mm long (N = 3); three lower facial horns not aligned, 0.04 mm long (N = 2); two pairs of lower facial papillae: one pair setose, the other bare; three pairs of lateral facial papillae: one pair setose,two bare;upper cephalic margin thickened laterally; face with pronounced lateral projection. Thorax: prothoracic spiracle 0.10 mm long (N = 2), as long as antennal basal width, setiform, slightly curved (N = 5) ( Fig. 20C View Figure20 ), integument wrinkled ( Fig. 20D View Figure20 ). Abdomen: segments 2-8 with transverse rows of crescent dorsal spines at basal half; posterior row with 26-30 spines in the 2 nd segment (N = 3), 24-31 in the 3 rd (N = 3), 24-29 in the 4 th (N = 3), 20-28 in the 5 th (N = 3), 20-22 in the 6 th (N = 3), 13-18 in the 7 th (N = 3), 7-10 in the 8 th (N = 3).
Larva ( Fig. 21A View Figure 21 ): Body: 2.20 mm long (N = 1); head retracted. Spatula ( Fig. 21B View Figure 21 ) quadridentate, 0.24 mm long (n = 1), lateral and mesal teeth subequal in length ( 0.02 mm long) (N = 1), lateral teeth more pointed than mesal; three setose lateral papillae on each side of spatula. Terminal segment smashed.
Gall: on fruit, globoid, green, glabrous, multichambered on Heliotropium sp. ( Heliotropiaceae ) ( Fig. 21C View Figure 21 ).
Material examined: Holotype male, BRAZIL, Rio de Janeiro, Mangaratiba, Ilha da Marambaia, Praia Grande , 16.XII.2009, A. R. Rodrigues leg. ( MNRJ-ENT1-69833 ) . Paratypes: FEMALES – Praia do Kutuca , 18.VII.2010: 2 ♀♀ ( MNRJ-ENT1-69834 , MNRJ-ENT1-69835 ) ; PUPAL EXUVIAE – same data as holotype, 1 pupal exuvia ( MNRJ-ENT1-69837 ) ; Praia do Kutuca , 18.VII.2020: 2 pupal exuviae ( MNRJ-ENT1-69838 ) ; PUPA – 1 pupa ( MNRJ-ENT1-69836 ) ; THIRD INSTAR LARVA – Praia do Kutuca , 25.II.2011: 1 larva ( MNRJ-ENT1-69839 ) .
Etymology: The species is named in honor of Dr. Roberto Xerez (Universidade Federal Rural do Rio de Janeiro) responsible for collection permission in the locality-type (area of the Brazilian Navy).
Geographic distribution: Brazil, Rio de Janeiro State, Mangaratiba municipality (Rodrigues et al., 2014).
Remarks: There is only one previously known species of Asphondylia on Heliotropiacae, A. tournefortiae Rübsaamen 1915 on Heliotropium angustiflorum (Ruiz & Pav.) Govaerts (reported as Tournefortia angustiflora Ruiz & Pav. ) and Myriopus volubilis Small (reported as T. volubilis L) from Brazil.
Adults of Asphondylia xerezi Maia , sp. nov. have longer body and shorter scape, pedicel and flagellomeres ( Table 5) than in A. tournefortiae . The larval spatula of A. xerezi is longer than in A. tournefortiae Rübsaamen, 1915 . In addition, mesal and lateral teeth are subequal in length the new species, while in A. tournefortiae mesal teeth are shorter than lateral ones ( Fig. 21B View Figure 21 × Fig. 21D View Figure 21 ).
amen1915 and A.xerezi Maia , sp.nov. ( Diptera , Cecidomyiidae ).Data on the first species were obtained from literature. Caires,C.S. & Dettke, G.A. 2023. Struthanthus .In: Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available: https://floradobrasil.jbrj.gov.
br/FB8703. Access: 12/02/2023.
Carvalho-Fernandes,S.P.; Ascendino,S.; Maia,V.C. & Couri, M.S. 2016. Diversity of insect galls associated with coastal shrub vegetation in Rio de Janeiro,Brazil. Anais da Academia Brasileira de Ciências, 88 ( 3): 1407-1418. https://doi.org/10.1590/0001-3765201620150658.
Coelho, M.S.; Almada, E.D.; Fernandes, G.W.; Carneiro, M.A.A.; Santos, R.M.; Quintino, A.V. & Sanchez-Azofeifa, A. 2009. Gall inducing arthropods from a seasonally dry tropical forest in Serra do Cipó,Brazil. Revista Brasileira de Entomologia, 53 ( 3): 404-414. https://doi.org/10.1590/S0085- 56262009000300015.
Gagné, R.J. 1994. The gall midges of the Neotropical region. Ithaca, Cornell University Press, 352p.
Gagné, R.J. & Jaschhof, M. 2021. A Catalog of the Cecidomyiidae (Diptera) of the World. 5.ed.Digital. 813p.
Lorenzi, H. & Matos, F.J.A. 2008. Plantas medicinais no Brasil. Nova Odessa, Instituto Plantarum. 544p.
Maia,V.C. 2001. The gall midges (Diptera, Cecidomyiidae) from three restingas of Rio de Janeiro State, Brazil. Revista Brasileira de Zoologia, 18 ( 2): 305-656. https://doi.org/10.1590/S0101-81752001000200028.
Maia, V.C. 2013. Galhas de insetos em restingas da região sudeste do Brasil com novos registros. Biota Neotropica, 13 ( 1): 183-209. https://doi. org/10.1590/S1676-06032013000100021.
Maia,V.C. 2021. Cecidomyiidae (Diptera,Insecta):richness of species and distribution in Brazil. Biota Neotropica, 21 ( 2): 1-35,e20201038. https://doi. org/10.1590/1676-0611-BN-2020-1038.
Maia, V.C.; Cardoso, J.L.T. & Braga, J.M.A. 2014. Insect galls from Atlantic Forest areas of Santa Teresa, Espírito Santo, Brazil: characterization and occurrence. Boletim do Museu de Biologia Mello Leitão, 33: 47-129.
Melo, J.I.M. 2023. Heliotropium. In: Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available: https://floradobrasil.jbrj.gov.br/FB16538. Access:09/03/2023.
Miller, J.S. 2013. A revision of Cordia section Gerascanthus (Boraginales: Cordiaceae). Journal of the Botanical Research Institute of Texas, 7: 55-83.
Miller, J.S. & Gottschling, M. 2007. Generic classification in the Cordiaceae (Boraginales): resurrection of the genus Varronia P. Br. Taxon, 56 ( 1): 163-169. https://www.jstor.org/stable/25065747.
Möhn, E. 1959. Gallmücken (Diptera, Itonididae) aus El Salvador. 1. Teil. Senckenbergiana Biologica, 40 (5/ 6): 297-368.
Möhn, E. 1960. Gallmücken (Diptera, Itonididae
) aus El Salvador. 2. Teil. Senckenbergiana Biologica, 41(3/4):197-240.
Rodrigues, A.R.; Maia, V.C. & Couri, M.S. 2014. Insect galls of restinga areas of Ilha da Marambaia, Rio de Janeiro, Brazil. Revista Brasileira
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.