Echinoderes cf. unispinosus Yamasaki, Neuhaus & George, 2018
|
publication ID |
https://doi.org/10.5852/ejt.2018.456 |
|
publication LSID |
lsid:zoobank.org:pub:DE1B1DEE-9871-4803-9F67-025F2B439560 |
|
DOI |
https://doi.org/10.5281/zenodo.3818834 |
|
persistent identifier |
https://treatment.plazi.org/id/F64287A2-506B-FFDF-1795-FED5FE900AF9 |
|
treatment provided by |
Valdenar |
|
scientific name |
Echinoderes cf. unispinosus Yamasaki, Neuhaus & George, 2018 |
| status |
|
Echinoderes cf. unispinosus Yamasaki, Neuhaus & George, 2018 Figs 24–25 View Fig View Fig , Tables 16–17
Material examined
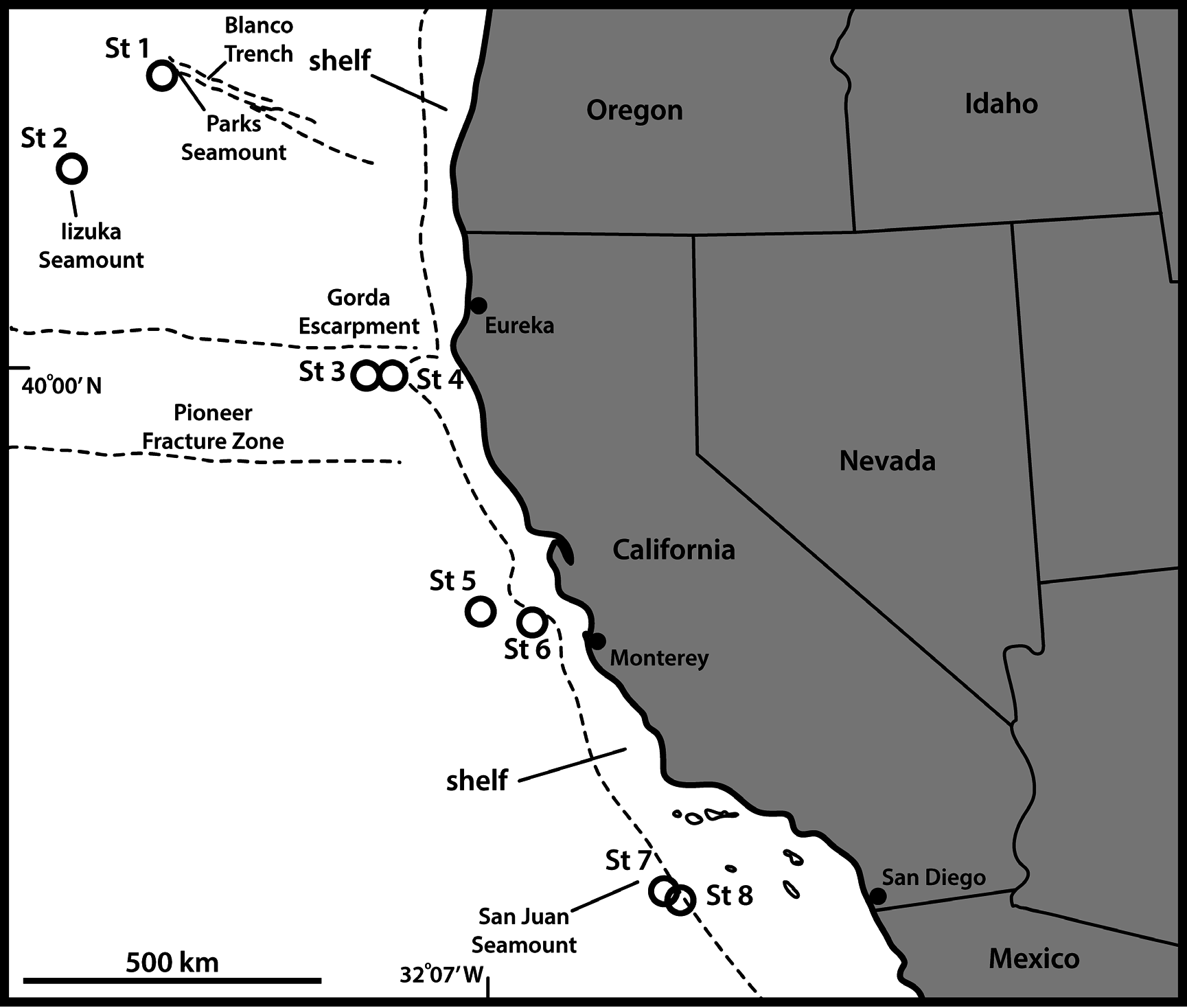
UNITED STATES OF AMERICA: 2 adult ♂♂, St. 1; 2 adult ♀♀, 1 adult ♂, St. 3; 3 adult ♀♀, 1 adult ♂, St. 4. All specimens mounted in Fluoromount G (NHMD-227016–227024). See Fig. 1 View Fig for localities and Table 1 View Table 1 for detailed station data .
Other material
UNITED STATES OF AMERICA: 3 ♂♂, St. 3; 1 ♂, St. 5. All mounted for SEM and stored in the first author’s personal reference collection .
Notes on distribution, morphology and comparison with type material
A complete overview of measurements and dimensions is presented in Table 16, and the distribution of cuticular structures, i.e., sensory spots, glandular cell outlets and spines is summarized in Table 17 View Table 17 .
Echinoderes unispinosus was recently described from the deep-sea plains around Sedlo Seamount in the Northwest Atlantic, north of the Azores, at a depth of 2875 m ( Yamasaki et al. 2018b). Besides being among the first bathyal species of Echinoderes to be described, E. unispinosus shows several unusual characters that make it highly distinct among congeners. These include the single middorsal spine on segment 4 ( Figs 24D View Fig , 25G View Fig ) and lateroventral spines on segments 6 and 7 only ( Figs 24E View Fig , 25 View Fig H–I), combined with the presence of glandular cell outlets type 2 on segments 1, 2, 5 and 8 ( Figs 24 View Fig B–C, E–F, 25C, E–F, H–J). As stressed by Yamasaki et al. (2018b) this species is distinguished very easily by its characteristic spine and outlet pattern.
The material examined for the present study yielded specimens with a very similar morphology ( Figs 24–25 View Fig View Fig ). Their general appearance is identical with that of E. unispinosus , their meristic data is within the range or very close to those of the type specimens (compare Table 16 with Yamasaki et al. 2018b), and all taxonomically significant characters, i.e., spines and glandular cell outlets type 2, fit E. unispinosus perfectly. The only observed differences between the Pacific specimens from the present study and the Atlantic specimens from the type locality are: sublateral sensory spots on segments 3 and 9 ( Fig. 25J View Fig ), observed in the Pacific population only, subdorsal sensory spots on segment 8, observed in the Atlantic population only, and sensory spots on segments 5 to 7 which occur midlaterally in the Atlantic population, and more sublaterally in the Pacific population ( Fig. 25I View Fig ).
The identity of the Pacific population leaves a taxonomic challenge. On the one hand, even though we can observe minor differences, these are so few and insignificant that they can hardly justify the description of a new species. Furthermore, Yamasaki et al. (2018b) did not have specimens available for
SEM and the specimens they had for LM were not in an optimal condition. Hence, it cannot be ruled out that they have missed a sensory spot, or misinterpreted another structure and considered it to be a sensory spot. Visualizing sensory spots in LM can be extremely challenging, especially in mid- and sublateral positions. On the other hand, no other species of Echinoderes shows a distribution as wide as this. Even though a species like Echinoderes tchefouensis Lou, 1934 is known to have a considerable distribution, and can be found throughout East Asia and Indonesia ( Sørensen et al. 2016b), its distribution still covers a smaller geographic area than the one suggested for E. unispinosus , and the distribution of
E. tchefouensis appears much more continuous, with numerous records of the species throughout its distributional range (Lou 1934; Higgins & Kristensen 1988; Sørensen et al. 2012; 2016b). As for the Pacific record of E. unispinosus , this leaves us with two options: 1) deep-sea species of Echinoderes might show a much wider distributional range than congeners from more shallow water. This option cannot be ruled out completely, since our knowledge of deep-sea Echinoderes is extremely limited, and has not been addressed until very recently ( Yamasaki et al. 2018a, 2018b; Grzelak & Sørensen in press). Alternatively, 2) the Pacific population represents a different species, closely related with E. unispinosus , but with morphological differences restricted to a few sensory spots. We might here feel inclined to support the second option, mostly based on the geographic distance between the two populations, but then again, we would hesitate in providing a formal description of the Pacific population as a distinct species, based on the present material. The question could perhaps be solved in the future if more material, and in particular clean specimens for SEM, were made available from the type locality of E. unispinosus , but ideally the problem should be approached by a molecular comparison of the two populations.
Table 17. Summary of nature and location of sensory spots, glandular cell outlets, tubes and spines arranged by series in Echinoderes cf. unispinosus. Abbreviations: LA = lateral accessory; LD = laterodorsal; LV = lateroventral; MD = middorsal; ML = midlateral; PD = paradorsal; PV = paraventral; SD = subdorsal; SL = sublateral; VL = ventrolateral; VM = ventromedial; ac = acicular spine; gco1/2 = glandular cell outlet type 1/2; ltas = lateral terminal accessory spine; lts = lateral terminal spine; pe = penile spines; si = sieve plate; ss = sensory spot; (♀) = female and (♂) = male conditions of sexually dimorphic characters; * = marks characters differing in presence/absence from the original species description of Yamasaki et al. (2018b); (*) = marks characters also present in the original species description of Yamasaki et al. (2018b), but in a slightly different position.
| Position | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment MD | PD | SD | LD | ML | SL | LA | LV | VL | VM | PV | |
| 1 | gco1 | ss | ss | gco2 | gco1 | gco1 | |||||
| 2 | gco1, ss | gco2 | gco2, ss | gco2 | gco2 | ss | |||||
| 3 | gco1 | ss | ss* | gco1 | |||||||
| 4 | ac | gco1 | gco1 | ||||||||
| 5 | gco1 | ss | ss (*) | gco2 | gco1, ss | ||||||
| 6 | gco1, ss | ss (*) | ac | gco1, ss | |||||||
| 7 | gco1 | ss | ss (*) | ac | gco1, ss | ||||||
| 8 | gco1, ss | no ss* | gco2 | gco1, ss | |||||||
| 9 | gco1, ss | ss | ss* | si (*) | ss | gco1 | |||||
| 10 | gco1, gco1 | ss | ss | gco1 | |||||||
| 11 | gco1, gco1 | ss | 3 x pe(♂) | ltas( ♀) | lts | ||||||
| MD |
Museum Donaueschingen |
| PD |
Dutch Plant Protection Service, Culture Collection of Plant Pathogenic Bacteria |
| ML |
Musee de Lectoure |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |