Tatia gyrina ( Eigenmann & Allen, 1942 )
|
publication ID |
https://doi.org/10.1590/S1679-62252008000300022 |
|
DOI |
https://doi.org/10.5281/zenodo.17315679 |
|
persistent identifier |
https://treatment.plazi.org/id/AD092F4C-FFDB-FF9B-FC48-15E4A3195925 |
|
treatment provided by |
Carolina |
|
scientific name |
Tatia gyrina ( Eigenmann & Allen, 1942 ) |
| status |
|
Tatia gyrina ( Eigenmann & Allen, 1942) View in CoL
Figs. 2-5 View Fig View Fig View Fig View Fig , 25-26 View Fig View Fig
Centromochlus aulopygius View in CoL . Eigenmann & Eigenmann, 1888: 157 [Cudajas (Codajás)]. Eigenmann & Eigenmann, 1890: 270 [distribution]. Eigenmann & Eigenmann, 1891: 34 [listing].
Centromochlus gyrinus Eigenmann & Allen, 1942: 118 View in CoL , pl.5 fig. 4 [ type locality: Peru, Iquitos, brook near Itaya river]. Gosline, 1945: 10 [listing]. Fowler, 1945a: 62 [Iquitos]; Fowler, 1945b: 112 [listing]; Fowler, 1951: 462, fig. 491 [literature compilation].
Centromochlus creutzbergi Boeseman, 1953: 7 View in CoL , fig. 1c [ type locality: Djaicreek (Djao stream)]. New synonym. - Hoedeman, 1957:151, fig. 8 [Coropina stream near Republiek].- Rössel, 1962: 30 [no locality].- Hoedeman, 1968: 148 [ Suriname].
Tatia gyrina View in CoL . Mees, 1974: 74-75 [notes on holotype and distribution]. Ortega & Vari, 1986:14 [reference]. Burgess, 1989:242 [reference]. Soares-Porto, 1995:205 [citation]. Soares-Porto, 1998: 333 [citation]. Ferraris, 2003:477 [checklist]. Ferraris, 2007: 77 [checklist].
Tatia creutzbergi View in CoL . Mees, 1974: 77-80, fig. 18 [notes and distribution, northern Suriname lowlands and Cudajas (=Codajás), Brazil]. Sands, 1984: 40 [listing]. Mees, 1985: 241 [northern Suriname]. Mees, 1988: 410 [ Para stream and Kaboeri stream, Suriname]. Burgess, 1989:242 [reference]. Soares-Porto, 1995:205 [citation]. Chang & Ortega, 1995:4 [reference]. Soares-Porto, 1998: 333 [citation]. Ferraris, 2003:476 [checklist]. Ferraris, 2007: 77 [checklist].
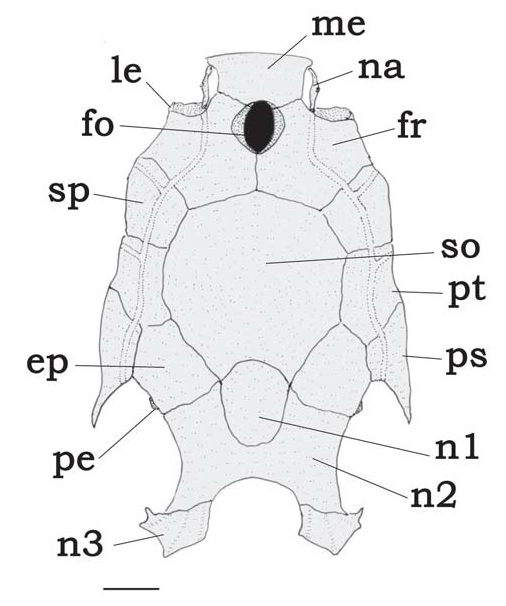
Diagnosis. Tatia gyrina is distinguished from all other species of Tatia by its reduced mesethmoid, wider than long, (mesethmoid length 12.1-15% HL; width 17.2-19% HL); lower jaw slightly protruding beyond upper; nasal ossified, tubular, not sutured to mesethmoid; third nuchal plate reduced ( Fig. 3, n3 View Fig ); small prevomer, with short rostro-lateral process; elongate postcleithral process, sometimes as long as head length; ribs 5-6 ( vs. 7-11 in other Tatia ); and vertebrae, 29-30 ( vs. 31-39 in other Tatia , except T. galaxias , that rarely has 30). Mature males have an externally elongate deferent duct and a swollen anal-fin base, with a thick secretory pouch ( Fig. 26 View Fig , tp). The species is also distinguished by the following combination of restricted characteristics: females with genital papilla; anal fin of mature males bearing rays with uncurved segments; and caudal fin lobes with about the same length in both adult females and males. Additional features useful for distinguishing T. gyrina has a whitish ground color pattern with brown spots, pattern mottled or with irregular stripes; ventral surface of head covered with short brown irregular stripes; body with a characteristic dark band along lateral line; and a small adult size of less than 40 mm SL.
Description. Measured adult specimens 28.0- 38.2 mm SL; morphometric data presented in Table 8 View Table 8 . Body short, head slightly depressed dorso-ventrally. Head large, robust, outline of head in dorsal view somewhat rectangular, broader than long. Dorsal outline of trunk from dorsal-fin base to caudal peduncle gradually compressed posteriorly. Lateral profile of head from snout tip to above opercular margin slightly convex until pectoral-fin insertion. Ventral profile of head and abdomen flat. Ventral profile of body concave posterior to anal fin.
Head integument thin, cranial roof visible; well-developed adipose eye lid; eye latero-dorsally located in anterior portion of head; mouth terminal, lower jaw slightly protruding beyond upper, upper lip extended postero-laterally as well-developed fleshy rictal fold; snout margin rounded, in dorsal view; anterior nostril tubular, located on anterior border of snout; posterior nostril large, rounded, limited by well developed skin flap; transverse distance between anterior nostrils shorter than distance between posterior ones. Maxillary barbel of moderate size, extending beyond opercular membrane, reaching vertical through middle dorsal fin; Four mental barbels, tips not reaching pectoral-fin base, arranged in arc along ventral surface of jaw; innermentalbarbelabout 50.0-67.0% lengthofoutermentals. Postcleithral process well developed, almost reaching vertical through middle or end of dorsal fin. Caudal peduncle moderately deep, its depth about 11.3-13.2% SL.
Rostral border of cranium thin with small mesethmoid, larger than long; premaxilla underneath with synchondral articulation; cranial fontanel large, rounded, bounded by mesethmoid and frontal ( Fig. 2 View Fig ); nasal ossified as short tubular canal bone, lying between mesethmoid cornua and lateral ethmoid, not sutured to mesethmoid; autopalatine tubular, oriented obliquely to longitudinal axis of body; maxilla small, shorter than autopalatine; prevomer reduced, with short lateral process. Discrete prognathous mandibula; premaxilla and dentary with two to three rows of conical teeth. First nuchal plate trapezoid; second nuchal plate short; third nuchal plate short, with tips projected laterally. Epioccipital process very small.
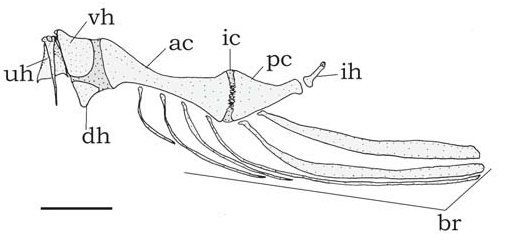
Suspensorium and opercular bones ( Fig. 2 View Fig ); hyoid arch ( Fig. 4 View Fig ) and branchial skeleton ( Fig. 5 View Fig ) as in generic description. Suprapreopercle present as short canal bone. Five to seven slender branchiostegal rays articulated with hyoid arch: three to five with anterior ceratohyal and two with posterior ceratohyal; last one expanded. Basibranchial 2 forming osseous rod with broad cartilaginous anterior tip, separated from shorter basibranchial 3.
Five to six infraorbital bones in incomplete series. Infraorbital 1 thin, with short ventro-lateral process; remaining infraorbitals thin, reduced to canalicular portions. Infraorbital 2 and/or 3 small, close to infraorbital 1, followed by nonossified portion of canal below eye and two posterior canal bones, forming posterior orbital rim. Lateral line on body with 3-5 ossified canal bones only close to head.
Dorsal fin I,3-5 (n=19), usually 4 (three branched only in T. creutzbergi holotype and five branched in oneAMNH 105.832 specimen). Dorsal-fin spine with 10-15 antrorse serrations along entire anterior margin, posterior margin smooth. Pectoral fin I,4 (n=21); pectoral-fin spine with 17-23 antrorse serrations along anterior margin; 9-13 retrorse serrations along posterior margin; serrations along both margins progressively larger towards spine tip. Pelvic fin i,5 (n=21); margin rounded. Adipose fin large, origin on vertical through middle of analfin base. Anal fin ii-iii, 6-7 (n=21); anal-fin pterygiophores in eight rod-like proximal radials and seven cartilaginous distal radials. Caudal fin slightly emarginated, lobes with rounded tips, 8+9 principal rays, 8-20 upper procurrent, 10-21 lower procurrent rays (n=21). Pleural ribs 5-6 attached to consecutive vertebrae. Post-Weberian vertebrae 29-30 (n=9).
Color in alcohol. Illustration in Eigenmann & Allen (1942: 439, fig. 4), shows holotype of T. gyrina as originally mottled brown, without dark lateral band, and specimen described as spotted and streaked ( Eigenmann & Allen, 1942: 118). Preserved holotype now faded and, based on recent examination of specimen, has only traces of pigmentation, consisting of small brown chromatophores on dorsal and caudal fins. Species coloration described as follows ( Fig. 25 View Fig ): Dorsal surface of head and dorsal fin dark brown; head irregularly striped in brown ventrally; sides of body mottled with numerous irregular spots; usually dark brown stripe along lateral line; body usually yellowish ventrally; pectoral-fin spine usually with transverse dark bands; adipose fin pale brown; caudal fin spotted with dark band on center of each lobe. Sands (1984: 40) illustrated a living specimen of T. gyrina .
Color variation. Variation in body pigmentation was observed between the Suriname and Amazonian populations. In Suriname specimens the body is usually yellowish ventrally or sometimes with a rounded brown spot present near each pelvic-fin base. No Amazon specimens had a rounded brown spot near the pelvic-fin base and the body is invariably yellowish ventrally. Variation in pectoral-fin spine pigmentation occur independent of geographical locality: pectoral-fin spine is usually transversely banded but is uniformly dark to unpigmented in some specimens. Coloration of the dark lateral stripe on the side of the body varies. In some Suriname specimens (ZMA 105.832) the dark brown lateral stripe has irregular dark and light areas. In addition, the lateral stripe was interrupted in some and absent in decolorized specimens. In an upper Amazon specimen (FMNH 94752), the lateral stripe is light brown, not as dark as in the Amazon specimens.
Tatia gyrina occasionally occur together with T. strigata and in these situations the two species are remarkably similar in appearance. The situation is exacerbated by the fact that T. strigata taken with T. gyrina have a dark lateral stripe, a character otherwise rare in T. strigata . In these instances, the main way to distinguish the two species is by certain head measurements. Tatia gyrina has a deep (snout depth 50.0- 59.4% HL), and broad head (head width 82.4-94.2% HL) and prognathous lower jaw; whereas T. strigata has a shallow (snout depth 41.3-47.3% HL) and narrow head (head width 70.1-76.7% HL), and jaws of equal sizes. There are also some minor color differences. The sides of body in T. gyrina are mottled, with variations in form and size of the individual spots, but never striated or striped like in T. strigata . Furthermore in T. strigata the stripes are long, almost horizontal and narrow.
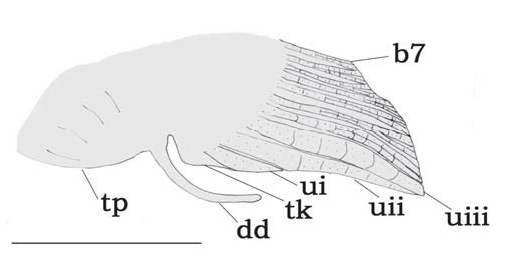
Sexual dimorphism. Based on examination of gonads, T. gyrina attains sexual maturity above 28.0 mm SL. In mature females a genital papilla is evident, with the genital opening close to the anal-fin origin, emerging from a slender fleshy process.A female genital papilla is rare within Tatia . The genital papilla of mature male is evidenced by a swollen anal-fin base ( Fig. 26 View Fig ). Dissection revealed the presence of a thick pouch inside it, formed by broad mass of some glandular tissue, with secretory material.A unique male deferent duct is present, externally elongate, thick and emergent ( Fig. 26 View Fig , dd). The extended deferent duct is also seen in young males, permitting the distinction between sexes even in juveniles. This condition is uniquely found in T. gyrina males. In other Tatia , as well as in most centromochlins, only short slender tip of deferent duct is externally visible, emerging from genital papilla.
The mature male modified anal fin ( Fig. 26 View Fig ) is short, with a small tip, and three enlarged and thickened unbranched analfin rays. First unbranched ray with the segments fused or bearing a single median one in one specimen (CAS 36979), and is immediately preceded by a short tegumentary keel ( Fig. 26 View Fig , tk). The second and third unbranched rays are converging with first branched to form a short anal-fin tip ( Fig. 26 View Fig , uiii). No curved segments are associated with the anterior rays. The posterior branched rays are normally developed, with the last ray not reduced ( Fig. 26 View Fig , b 7 View Fig ).
Hemal spines 13-16 interdigitate with the anal-fin pterygiophores, being the hemal spines 14-16 thickened in mature males, but undifferentiated in females. Minor sexual dimorphism is observed in male caudal fin: upper lobe of some males is slightly elongate, about 10% longer than lower one. The caudal-fin lobes have same length in mature females.
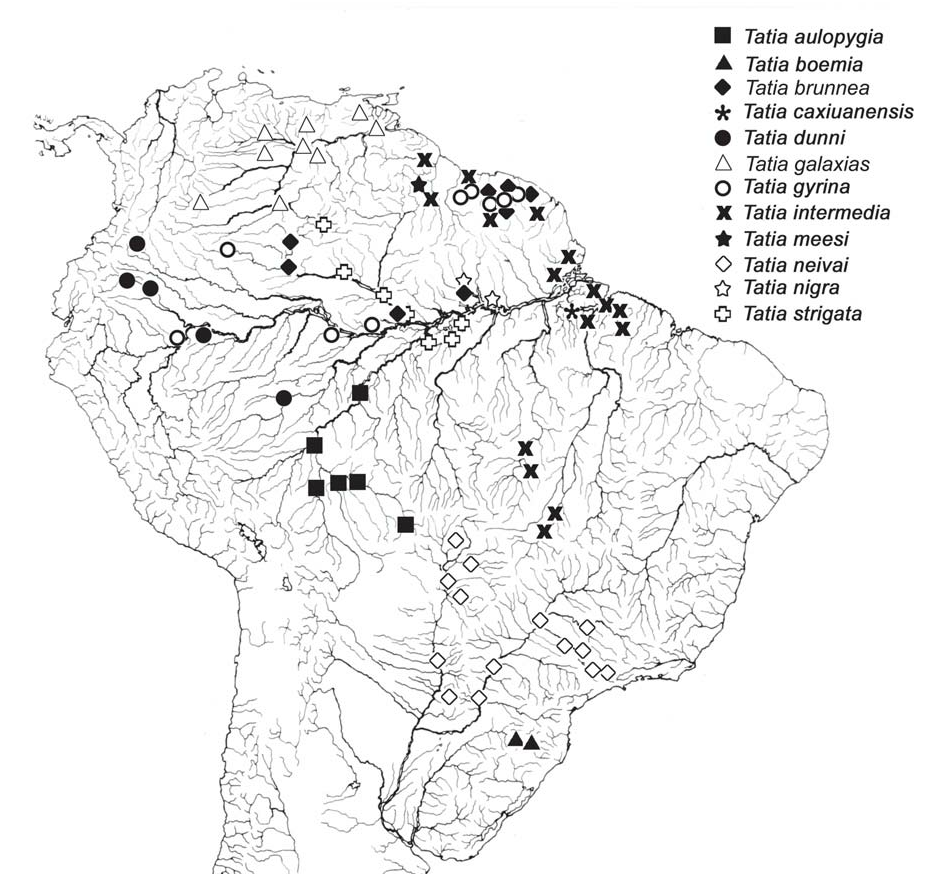
Distribution. Tatia gyrina occurs in the upper and central Amazon, in Peru, Colombia and Brazil. It is also found in rivers of northern Suriname ( Fig. 6 View Fig ).
Remarks. This species was originally described by Eigenmann & Allen (1942) as Centromochlus gyrinus based on a single specimen from Iquitos, Peru. Nearly a decade latter, Boeseman (1953) described Centromochlus creutzbergi based on a specimen from Suriname, but without a comparison to Centromochlus gyrinus . Mees (1974) transferred both species to the genus Tatia , based on similarities in fin ray counts and the shape of adipose fin. For many years the Amazonian T. gyrina was known only from its holotype. The holotype of T. gyrina is old and has lost its color. The specimen, however, is a mature male, with a modified anal fin that is similar in appearance to the anal fin of specimens from Suriname identified as T. creutzbergi . Recent sampling of Amazon igarapés has made available more specimens, including adults, what has allowed for a more detailed morphological comparison of Amazon and Suriname populations. The new material indicates that T. creutzbergi from Suriname and T. gyrina from the Amazon are morphologically very similar and hereby considered conspecific under the older name T. gyrina . Differences generally associated with color pattern and certain head proportions are considered intraspecific geographic variation. Both have the same general coloration, a conspicuous dark lateral stripe and mottling on sides of body. In the Amazon populations, however, the dark stripe is interrupted in some specimens, whereas in all Suriname specimens examined the dark stripe is always present and conspicuous. In some Suriname specimens the pelvic-fin base has a characteristic darkened area, with paired brown spots. These markings are especially obvious in specimens recently captured. Some Suriname specimens have the ventral surface of body uniformly whitish, (ZMA 105.829), in the same way asAmazon specimens. The low number of vertebrae, 29 in Amazonian catfishes (n=3), and 30 in Suriname ones (n=6), was counted in some specimens only, and overlap of counts may occur. The Amazon and Suriname populations of T. gyrina also exhibit a few differences regarding head proportional measurements: head slightly broader and deeper in Amazon specimens, 88.8-94.2% and 56.3-59.4% HL, respectively ( vs. narrower and shallower in Suriname, 82.4-86.7% and 50.0- 55.4% HL, respectively); and mouth wider in Amazon specimens, 56.6-61.9% HL( vs. narrower in Suriname 40.2-45.6% HL). Regional differences in head measurements and vertebral counts are interpreted as intraspecific variation within T. gyrina .
Tatia gyrina is typically associated with blackwater environments, mainly in rivers. In the central Amazon, populations of T. gyrina were found in both black- and clear-waters, but only in dense vegetation where there is little light (J. Zuanon, pers. comm.). Available field data indicate these catfish typically occur in water with low pH, low conductivity and low level of dissolved oxygen (J. Zuanon, pers. comm.; H. Nijssen, field notes).
Material examined. 80 specimens ( 23.6-38.2 mm SL). Holotype. Peru: Loreto: CAS 36979 , 1 (42.0 mm SL), tributary of Itaya river , above Iquitos (holotype of Centromochlus gyrinus ). Suriname: RMNH 19440 , 1 (R) ( 25.2 mm SL), Djaicreek (holotype of Centromochlus creutzbergi ) . Non-type specimens. Brazil: Amazonas: MCZ 8182, 1 ( 32.7 mm SL), Brazil:Amazonas: Solimões river in Codajás. INPA 18478, 1 ( 38.2 mm SL), Brazil. Amazonas: Tefé. INPA 20970,2 (29.0- 36.2 mm SL), Tefé, reserva desenvolvimento sustentável Amanã, igarapé Branco. INPA 20971, 4, 1 CS (26.0- 28.3 mm SL), Tefé: reserva de desenvolvimento sustentávelAmanã, igarapé do Veado. INPA 20972, 1 ( 35.1 mm SL). Colombia: FMNH 94752, 1, Ti river. Suriname: AMNH 58389, 2 ( 28.6-29.7 mm SL), Carolina stream. RMNH 28616, 5 ( R) (31.0-33.0 mm SL), Sipaliwini: upper Nickerie river; RMNH 28617, 10 (1 CS) (25.0- 34.1 mm SL), Sipaliwini: tributary on right margin of Nickerie river, above Stondansie Camp; RMNH 28618, 8 (31.2-35.0 mm SL), tributary on right margin of Fallowatra river, Nickerie river basin; RMNH 28619, 7 (26.3- 34.0 mm SL), Forest stream, above Stondansie, Nickerie river basin; RMNH 28620, 14 ( 30.4-37.2 mm SL), tributary on left margin of Nickerie river, above Blanche Marie falls; RMNH 28621, 1 ( 34.7 mm SL), small tributary of middle Maratakka river; RMNH 28622, 2 (28.2-30.0 mm SL), Winanna river; RMNH 28623, 2 (14.5-21.0 mm SL), tributary of Kabalebo stream, below Avanavere falls; RMNH 30540, 1 ( 26.2 mm SL), Kaboeri stream; ZMA 105.523, 1 (39.0 mm SL), Gran Mau stream, right margin of the Gran river, 1 km northwestern from Village Dombaai; ZMA 105.657, 1 ( 33.6 mm SL), Saramacca river; ZMA 105.832, 4 ( 27.4-33.6 mm SL), Nickerie river; ZMA 105.859, 10 (1 CS) ( 23.1-30.1 mm SL), Para: Carolina stream.
| MCZ |
Museum of Comparative Zoology |
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
| CS |
Musee des Dinosaures d'Esperaza (Aude) |
| FMNH |
Field Museum of Natural History |
| AMNH |
American Museum of Natural History |
| RMNH |
National Museum of Natural History, Naturalis |
| R |
Departamento de Geologia, Universidad de Chile |
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tatia gyrina ( Eigenmann & Allen, 1942 )
| Sarmento-Soares, Luisa Maria & Martins-Pinheiro, Ronaldo Fernando 2008 |
Tatia gyrina
| Ferraris, C 2007: 77 |
| Ferraris, C 2003: 477 |
| Soares-Porto, L 1998: 333 |
| Soares-Porto, L 1995: 205 |
| Burgess, W 1989: 242 |
| Mees, G 1974: 74 |
Tatia creutzbergi
| Ferraris, C 2007: 77 |
| Ferraris, C 2003: 476 |
| Soares-Porto, L 1998: 333 |
| Soares-Porto, L 1995: 205 |
| Burgess, W 1989: 242 |
| Mees, G 1988: 410 |
| Mees, G 1985: 241 |
| Sands, D 1984: 40 |
| Mees, G 1974: 77 |
Centromochlus creutzbergi
| Hoedeman, J 1968: 148 |
| Rossel, F 1962: 30 |
| Hoedeman, J 1957: 151 |
| Boeseman, M 1953: 7 |
Centromochlus gyrinus
| Fowler, H 1951: 462 |
| Gosline, W 1945: 10 |
| Fowler, H 1945: 62 |
| Fowler, H 1945: 112 |
Centromochlus aulopygius
| Eigenmann, C 1891: 34 |
| Eigenmann, C 1890: 270 |
| Eigenmann, C 1888: 157 |